That staying reported, if you have to fill in some understanding gaps, don’t be afraid to request clarification!
My capacity to understand speedily and implement my awareness to different cases tends to make me an excellent prospect for just about any posture.”
Printed USFDA 483s (Inspectional observations issued on the shut of inspections) are reviewed and talked over focussing on what may lead to these observations, what you need to have in position to meet company expectations and prevent these types of observations at your production web site.
Qvents focusses on Pharma Top quality Occasions (Qvents), Regulatory observations and actions. You could be involved in discussions, lead your Thoughts and Views, Qvents is a robust medium where by your experience and know-how on the subject can appear alive, get peer reviewed & commented and get recognized…
My target will be to normally give Medical practitioners with the top tips and assist so which they will make informed choices for his or her individuals.”
Anyways, I’m saying you'll be able to clarify expectations having a, “How do you typically like subject matter discussions to go? Something Unique I ought to know beforehand?
Question: How can you cope with deviations from top quality benchmarks inside a pharmaceutical production placing?
I also fully grasp the importance of keeping up-to-day on adjustments for the regulatory surroundings And exactly how they are able to have an effect on products approvals. Lastly, I've encounter Operating carefully with interior teams to make certain all needed methods are taken to acquire FDA acceptance.”
“I've designed a number of strategies to stay organized whilst viewing multiple accounts every day. Very first, I create an agenda to the working day that outlines which accounts I want to visit and what duties I want to complete at every one. This can help me monitor my progress during the day and makes sure that I don’t overlook any essential specifics. Second, I use a customer romance administration (CRM) system to keep all of my notes from Every account to ensure that I can easily refer back again to them Sooner or later.
Continuous Enhancement: copyright supports continual improvement attempts by delivering actual-time details insights into approach functionality.
Qvents more info is usually a awareness sharing System focussed more info on Pharma Good quality Programs, GMP and Regulatory topics. Qvents discusses unique quality and regulatory gatherings, what will cause these types of functions, what businesses can perform to circumvent these situations.
USFDA Warning letters are reviewed, with Assessment of essential deficiencies and observations, what can lead to the observations, what you ought to have set up to meet company anticipations and prevent these types of observations and evets at your site, corporation.
Be knowledgeable about current issues and target areas of regulators and auditors; Share your views, feedback, hear from field peers….
I also Be sure to stay awake-to-day on marketplace trends and exploration so I can provide them with probably the most pertinent facts.
 Alicia Silverstone Then & Now!
Alicia Silverstone Then & Now! Barbi Benton Then & Now!
Barbi Benton Then & Now! Nancy McKeon Then & Now!
Nancy McKeon Then & Now!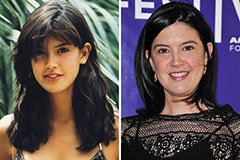 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Traci Lords Then & Now!
Traci Lords Then & Now!